Introduction
Blue litmus paper is a type of acid-base indicator used in chemistry to test for the presence of an acid or a base. The paper changes color when exposed to an acid or a base, indicating the level of acidity or alkalinity present in a solution. This article will explore the science behind blue litmus paper and provide a comprehensive guide to understanding and utilizing it in experiments.

Exploring the Science Behind Blue Litmus Paper
In order to understand how blue litmus paper works, it is important to first understand what makes up an acid or a base. Acids are molecules that donate hydrogen ions (H+) when dissolved in water, while bases are molecules that accept hydrogen ions when dissolved in water. Acids and bases can be identified by measuring the pH of a solution; pH is a measure of the concentration of hydrogen ions in a solution. A pH of 0-7 is classified as acidic, a pH of 7 is neutral, and a pH of 7-14 is basic or alkaline.
The effect of acids and bases on blue litmus paper is determined by the pH of the solution. If the pH of a solution is below 7, the solution is considered acidic and blue litmus paper will turn red. If the pH of a solution is above 7, the solution is considered basic and blue litmus paper will turn blue.

A Comprehensive Guide to Understanding Blue Litmus Paper
Now that we have explored the science behind blue litmus paper, let’s take a look at what it is and how to use it. Blue litmus paper is a piece of specially treated paper that contains a dye known as litmus. When exposed to an acid or a base, the litmus dye will turn either red or blue depending on the pH of the solution.
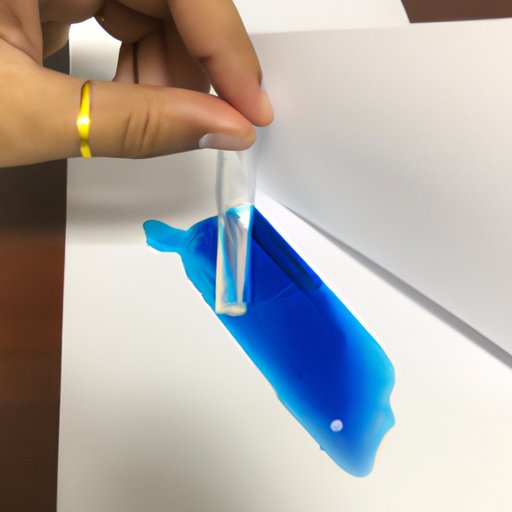
To use blue litmus paper, simply dip the paper into a solution and observe the color change. If the paper turns red, the solution is acidic. If the paper turns blue, the solution is basic. It is important to note that blue litmus paper should not be used to measure the exact pH of a solution; instead, it should be used as an indicator of whether a solution is acidic or basic.
How to Utilize Blue Litmus Paper in Experiments
Blue litmus paper can be used in a variety of experiments. When setting up an experiment with blue litmus paper, it is important to first establish a hypothesis. For example, if you were testing the acidity of a certain solution, your hypothesis might be that the solution is acidic. Once you have established your hypothesis, you can set up your experiment by gathering the necessary materials and preparing the solution.
Once your experiment is set up, you can use blue litmus paper to test the acidity or basicity of the solution. Simply dip the paper into the solution and observe the color change. If the paper turns red, the solution is acidic and your hypothesis is correct. If the paper turns blue, the solution is basic and your hypothesis is incorrect.
Deciphering the Properties of Blue Litmus Paper
When using blue litmus paper, it is important to understand its properties. One key property of blue litmus paper is its color change. As mentioned earlier, blue litmus paper will turn red when exposed to an acid and blue when exposed to a base. It is also important to note that the color change of blue litmus paper is reversible. This means that if the paper is exposed to an acid and turns red, it can be exposed to a base and will turn back to blue.
Comparing Red and Blue Litmus Paper: What’s the Difference?
Although both red and blue litmus paper are used to test for the presence of an acid or a base, there are some key differences between the two. The most obvious difference is their color change; red litmus paper will turn blue when exposed to a base, while blue litmus paper will turn red when exposed to an acid. Another difference is their uses; red litmus paper is typically used to test for the presence of an acid, while blue litmus paper is used to test for the presence of a base.
Conclusion
Blue litmus paper is a useful tool for identifying the presence of an acid or a base in a solution. By understanding the science behind blue litmus paper and how to utilize it in experiments, you can use this indicator to gain a better understanding of the acidity or alkalinity of a solution.
(Note: Is this article not meeting your expectations? Do you have knowledge or insights to share? Unlock new opportunities and expand your reach by joining our authors team. Click Registration to join us and share your expertise with our readers.)